Abstract
Introduction: Chimeric Antigen Receptor (CAR) T-cell therapy is now approved as a second-line treatment for relapsed/refractory diffuse large B cell lymphoma (DLBCL), multiple myeloma (MM), and acute lymphoblastic leukemia. B-cell maturation antigen (BCMA) and CD19 CAR-T therapies are associated with substantial hematological toxicity, potentially causing complications in the post-CAR-T setting. It is unclear if cytopenia rates differ between CAR-T therapies. We evaluated differences in cytopenia, transfusion support, and associated outcomes in patients receiving CAR-T therapy for MM and non-Hodgkin's lymphoma (NHL) at our institution.
Methods: We performed a retrospective analysis of patients diagnosed with MM and NHL from 2012-2022 who subsequently relapsed and received CAR-T-cell therapy at the Winship Cancer Institute at Emory University. Patients were excluded if they underwent apheresis but had not received infusion or had manufacturing failure. We compared rates of grade 3/4 cytopenias based on CIBMTR criteria and supportive care at day 0, 3mo, 6mo, and 1 year as well as date of hematological recovery. Day 0 was defined as onset of grade 3/4 cytopenia after CAR-T therapy. Hematological recovery was defined as sustained hemoglobin >8g/dL, platelets >50,000/µL, and ANC >1000/mm3 without need for support. Differences between groups were determined using Fisher's exact test and Kruskal-Wallis Test where appropriate. PFS and overall survival (OS) were estimated using the Kaplan-Meier method from CAR-T infusion to progression or death for those events and last contact for those censored. Statistical analysis was performed using SAS with significance at the 0.05 level.
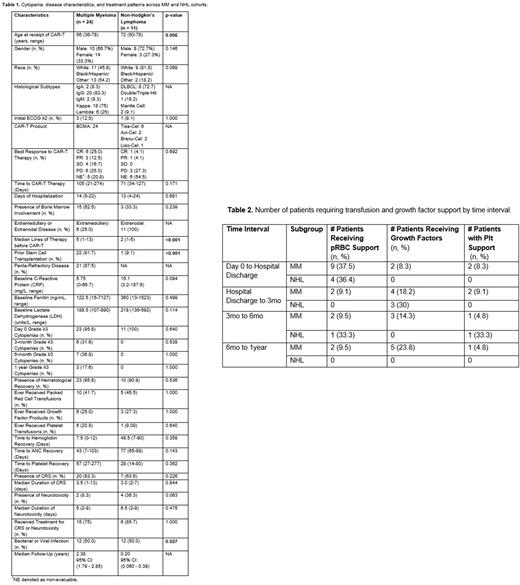
Results: Of 35 patients, 24 received BCMA and 11 received CD19 CAR-T therapy. Median age at CAR-T infusion for all patients was 58 years (36-78). Most patients were male (66.7%) and of white race (57.1%) [Table 1]. Overall, 4 patients (11.4%) had an initial ECOG ≥2. Twenty-one patients (87.5%) had penta-refractory MM disease with extramedullary involvement in 25%. All patients with NHL had stage 4 disease with bone marrow involvement in 33.3%. Eight patients had DLBCL (72.7%), 1 double hit lymphoma (18.2%), and 2 mantle cell lymphoma (9.1%). Six patients received tisa-cel, 2 axi-cel, 2 brexu-cel, and 1 liso-cel. The MM cohort underwent significantly more autologous transplantations as a prior line of therapy (p<0.001). Patients with NHL received CAR-T at a later median age (p=0.006). Median follow-up for the MM and NHL cohort was 2.38 years and 0.2 years respectively. There was no difference in time from last therapy to CAR-T infusion (p=0.171) nor days of hospitalization (p=0.681).
Almost all patients (95.8%) achieved hematological recovery after infusion with similar recovery rates between groups (p=0.536). Median time to grade 3/4 anemia recovery was 6 days (1-90), neutropenia recovery at 15 days (1-336), and thrombocytopenia recovery at 28 days (14-277). Time to grade 3/4 anemia, neutropenia, and thrombocytopenia was 1 day (0-11), 2 days (0-11) and 2 days (0-15) respectively. Most patients required supportive care with growth factors (34.6%), packed red cells (57.7%), and platelets (23.1%) without significant group differences [Table 2]. One patient with MM received TPO. Prolonged grade 3/4 cytopenia rates were significant with 17.1% at 3mo, 22.9% at 6mo and 8.7% at 1 year. Both groups had similar rates of grade 3/4 cytopenia at day 0, 3mo, 6mo, and 1 year.
The MM cohort had higher rates of infection (p<0.001). Rates of CRS (p=0.226) and neurotoxicity (p=0.063) were similar. Fourteen patients with MM (58.3%) and 3 patients with NHL (27.3%) progressed post-CAR-T. Median PFS overall was 1.7 years, 2 years (95%CI: 1, 3) in the MM group, and not reached (NR) (0.2, NA) in the NHL group. Median OS overall was NR, NR in MM (2.5, NA) and NR in NHL (0.3, NA). Grade 3/4 neutropenia and thrombocytopenia were not associated with survival at any time interval. Onset of grade 3/4 cytopenia were not associated with histopathological characteristics, inflammatory markers, or CRS/ICANS.
Conclusions: This real-world study demonstrates comparable rates of upfront and prolonged cytopenia in patients receiving BCMA and CD19 CAR-T therapies. Over 20% of patients had prolonged, ongoing grade 3/4 cytopenias requiring supportive care at the 3-6mo mark. Further follow-up and larger prospective studies are needed to validate these findings.
Disclosures
Romancik:AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Cohen:Lilly Oncology/Eli Lilly: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Janssen: Consultancy; Kite Pharma/Gilead: Consultancy; Aptitude Health: Consultancy; Takeda: Research Funding; Genentech: Research Funding; HutchMed: Consultancy, Research Funding; Astrazeneca: Consultancy, Research Funding; BMS/Celgene: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal